This work deals with the experience carried out by the Secretariat of Health and Social Medicine of the Municipality of La Plata and Mundo Sano Foundation from July 2010 to December 2013 at school institutions of the Buenos Aires province and primary health care centers of the municipality (CAPS). They are aimed at implementing a pilot trial to address the situation of people affected by Chagas disease from a comprehensive perspective, carrying out early diagnosis and timely treatment in a specific non-endemic geographic area of La Plata city, in the Buenos Aires province
Chagas in a Non-Endemic Area: First Level Health Care. Lights and Shadows
Mabel Lenardòn*, Patricio Orsini*; Marina Chopita*, Pedro Ramos*, Ana Paula Da Cruz*, Florencia Suárez Crivaro*, Sonia Tarragona**, Patricia Le Moal*, Belén Osaeta*, Jaime Henen*, Ana C. Pereiro**
**Mundo Sano Foundation
Introduction
Chagas Disease (CD) is named after Carlos Ribeiro Justiniano Chagas, a Brazilian physician that discovered it in 1909. The parasite causing the disease is Tripanosoma cruzi, which nests in several tissues and produces irreversible heart damage in 30% of chronic patients, and neurological and digestive injuries in 10 % of the cases. 1
In endemic areas, the most frequent form of transmission is vector-borne, through the bite of hematophagous insects called “vinchucas”. Likewise, there is evidence in the region of transmission through the intake of contaminated food and drinks. 2
There are three forms of transmission that are not related to any geographical area, namely: through transfusions, organ transplantation, and congenitally (mother to child). The first two are currently not frequent due to the presence of strict controls at blood banks. However, the congenital form (mother to child) constitutes a serious problem since it is the main form of transmission of the disease in non-endemic areas. As a result of migrations, women infected spread the infection to other areas congenitally, and what is even more worrying is that it is the least perceptible form to perpetuate the disease. 3–6
In the most usual forms of transmission, which are vector-borne and congenital, children are the most exposed, which makes CD a relevant pediatric issue for the countries in the region. 7-8 For this reason, the aim of this document is to present the results of a pilot program for detection and treatment of people affected by CD, especially school children and pregnant women in a semi-rural non-endemic area of the Buenos Aires province so as to assess the need to expand the test to cover all the district with a program with characteristics that are similar to those developed at the pilot trial, and, at the same time, analyze the operating capacity of the Primary Health Care Centers (CAPS) for this pathology.
Current State of Chagas Disease
Chagas Disease is characteristic of Latin America and according to recent PAHO estimates, in the Americas there are approximately 100 million people at risk, over 8 million people infected and approximately 56,000 new cases every year, who become infected through different forms of transmission. Around 12,000 people die of Chagas disease annually. 9
In Argentina, a prevalence rate at 4.1 is estimated for the general population, with over 1,600,000 infected people and variable regional percentages. The population exposed in endemic areas amounts to 7,300,000 people. 10-11
Even though significant progress has been made regarding control of vector transmission in the Americas, the lack of actions prolonged along time, aimed at eradicating Chagas disease from the endemic areas, has given rise to a continuous prevalence of this disease, whereas migrant phenomena have been the factor giving rise to its geographical expansion into urban areas, thus becoming a public health concern in nations where vector transmission has not been documented yet.
The Chagas disease figures available at present show a serious public health problem, although its magnitude is even greater due to the presence of important under-reporting of the number of cases. The reasons lying beneath said under-reporting can be found in the fact that in many places, the disease must not be compulsorily reported, or that only severe cases are reported, even when there are sub-clinical acute cases. Apart from that, since it presents no symptoms, the disease remains unnoticed by the people affected, who consequently do not seek for assistance; besides, detection demands the active search for positive cases, which is not frequent in most of the countries affected.
Justification of the Intervention
Approximately 750, 000 children are annually born in Argentina, 12 figure that makes us infer that the health system each year hosts between 34,000 and 45,000 pregnant women infected by Tripanosoma cruzi (according to estimated prevalence rates) and from 1,700 to 2,200 newborns with congenital Chagas disease.13 Currently, only 30% of these cases are diagnosed, which means that there will be thousands of children that will develop the disease during adulthood and, if they are girls, they will be likely to continue transmitting the infection to their own children if not timely treated.
Unfortunately, if diagnosis is made during pregnancy, the mother cannot be treated since the drugs available are contraindicated during the gestation period. Neither is there any efficient way to perform diagnosis of fetal infection during the intrauterine period, so the most adequate procedure at this stage consists of early diagnosis of newborns followed by specific treatment with beznidazole or nifurtimox, the only two drugs currently available for treatment of the disease. 14
Early detection of positive cases does not only bring about social and health benefits, but also economic ones.
Care of People Affected
Certainly, there are many reasons that can justify why this pathology had for so long been part of the list of neglected diseases. Without trying to go deeply into each of them, we will try to describe those that, to our belief, can explain most of the veil of neglect that covered Chagas disease for so long.
During the 70s, 80s and part of the 90s, Chagas disease occupied a limited space in physicians’ training curricula -pre-graduate reference books, mainly American and Spanish, did not develop the issue extensively- and specific treatments, particularly in the chronic phase, were challenged. All these factors together triggered a clear delay in diagnosis and treatment of the people affected, most of whom were under-diagnosed and/or disheartened to follow parasiticide treatments.
The lack of training and information of health teams could have had an influence on some of the behaviors observed such as that of considering Chagas a disease related to rurality and with no effective treatment, which discourages the implementation of mechanisms tending to start actions for primary, secondary and even tertiary prevention of the disease.
Devoting the health space to the exclusive care of the complications brought about by Chagas disease is to condemn many individuals to miss the opportunity to have timely treatment and lead a plentiful life.
The lack of symptoms for several years and even decades demands a proactive health system that can find outside the hospital sphere -the paradigm of the environment where the process health-disease takes place- those people who do not have the need to seek medical consultation. It is for that reason that it is necessary to implement epidemiological research as well as training and updating activities intended for the members of the primary health care teams, who are the access to the health care system, both strategies with different degrees of implementation.
The characteristics of the Argentine health system could also have had a negative influence on the comprehensive health care sometimes demanded by these patients, since there are multiple administrative areas that coexist in the same territory (provincial hospital-municipal CAPS and national institutions) which are not always integrated.
Relegating Chagas disease patients to exclusive treatment at the third level of care in order to receive treatment by specialist’s means, in many cases, imposing a barrier to access to the system since said treatment, for multiple reasons, is hard to be provided to people who have low resources and live in distant geographic regions.
Currently, there is consensus regarding the fact that patients with this disease can and must be followed at the first level of care, leaving the levels of increasing complexity to those who show moderate and/or severe complications. The present work offers a strategy (pilot trial), among many likely ones, to start these actions. 15-16
Methodology
In order to develop intervention models, the principles of stability, sustainability, scalability and replicability are taken into account. This means that our projects are permanent and sustainable along time, which means that they can be started in a reduced geographic area or with a small group of people (depending on the approach) so that once tested, they can be improved, expanded and replicated in another area or in other groups.
The first step is to perform a diagnosis of the situation that shows the state of affairs, then design and plan the most adequate type of intervention in this case, create strategic partnerships and working teams, manage the project with the local actors (community participation is key to the successful achievement of the projects) and finally, the processes and results are assessed and the necessary adjustments made.
In this pilot trial, a program for public health intervention was designed to perform diagnosis of Chagas disease on a school population in certain areas of La Plata district, Buenos Aires province. In order to detect positive Chagas Disease cases in an infant population and start timely treatment, the choice of the school environment as a recommended geographic area to develop these actions seems adequate, due to, among other reasons, the high level of schooling usually presented by the first level of education and the lower need of time and resources posed by this option. Likewise, the Primary Health Care Centers were used as the environment for detection, diagnosis and treatment of congenital Chagas disease, especially to attract the group of pregnant women there.
Selection of the area of intervention
The choice of province and area of work were based on the analysis of relevant variables to identify the population at potential risk and in terms of opportunity and access.
The analysis of the relevant variables consisted in surveying data produced by the Populational Censuses, the Permanent Household Survey (EPH after its name in Spanish) and the General Directorate of Migrations in relation to the 2004-2012 series of settlements issued by the National Directorate of Migrations and the analysis of domestic migrations on 2001 census data. The 24 regions of the Greater Buenos Aires area first, and then the Autonomous City of Buenos Aires are coincidentally presented there as the greatest jurisdictions that welcome domestic and foreign migrants coming from regions where CD is a public health issue (Bolivia, Paraguay, etc.). 17-18
Graph 1. Relevant variables used for selecting the intervention area
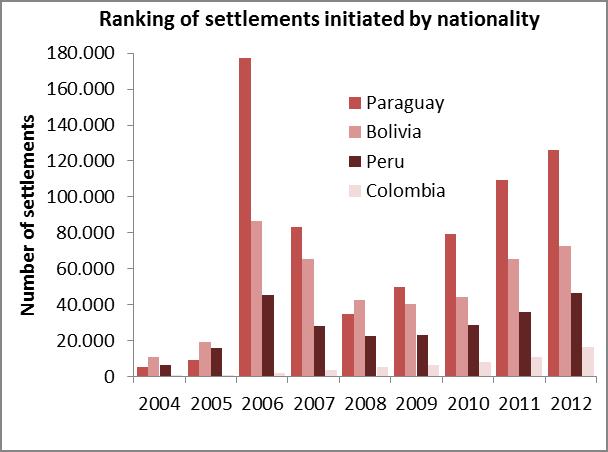
Source: Own elaboration based on the National Directorate of Migrations (2004-2012)
Given that the overall surface of the Buenos Aires province is over 300,000 km2, delimiting a geographic area was key to start the activities. To that aim, special attention was put on the following:
1. Data provided by professionals of the Urban and Rural Zoonosis Directorate of Buenos Aires province
2. The domiciles of the 181 women with positive serology for Chagas, who had given birth during the month of September 2008 and who were surveyed for perinatal risk factors as carried out by the Directorate of Maternity and Infancy of the Ministry of Health of the Buenos Aires province 19 and
3. Criteria of opportunity such as the willingness of the different districts to perform the assessment, distance to urban centers and health equipment and infrastructure available.
Finally, an area of land intervention in La Plata city, encompassing 190 km2, as well as an area for sanitary work encompassing 20 km2 were delimited.
Development of the Pilot Trial
The target population was selected pursuant to Section 4 of Law 26,281 -the new Law on Chagas Disease passed in 2007- which prioritizes school-age children and pregnant women and their children.
In order to carry out the activities of the pilot trial, a framework agreement was signed between the participating parties, the Secretariat of Health and Social Medicine of the Municipality of La Plata and Mundo Sano Foundation, and plans were made to perform interventions in the three schools located in semi-rural areas, and in three CAPS in their vicinity.
This first trial made it possible to establish a connection between the teams of professionals, the school community and the parents of the children attending those schools, as well as to test the design of the work. To that aim, a risk survey was outlined and delivered onto the school institutions, which evaluated the risk to suffer the disease as a consequence of the location where the mother and child had been born, of the blood transfusions received, and the positive serology for Chagas disease tested during pregnancy. All the variables were given the same relevance so as to determine the potential risk.
The scheme of activities contemplated the following steps:
1. Request an authorization to school supervisors and, once obtained, hold a meeting with the school authorities and teachers to explain to them the relevance of the work carried out, as well as the requisites needed to have said work accomplished,
2. Organize informative meetings with parents to explain the reach of the disease and the importance of early detection,
3. Deliver a risk survey and informed consents,
4. Assess the results,
5. Make appointments to see the children detected at risk and have their venous blood samples taken,
6. Refer the samples to the central laboratory to have them processed,
7. Make individual appointments with the parents of children with positive serological tests to explain to them the consequences of CD, inform them that the treatment is free of charge and introduce them to the health professional that will do a follow-up of the patient at the CAPS
8. Likewise, the need to assess other next of kin of those affected is communicated to them, and if they result positive, the same treatment and follow-up process will be carried out.
Once the pilot trial is finished, the necessary adjustments were made and work continued at other schools located on the perimeter mentioned, incorporating the survey and informed consent to the Program of Health at Schools (Programa de Sanidad Escolar-PROSANE), in force in the district.
At the same time, routine and systematic inclusion of Chagas disease serology was reinforced for all pregnant women at the CAPS, and members of the health team were trained in Chagas disease diagnosis and treatment. Likewise, women who resulted positive in their serology were called to be controlled and treated after delivery; family planning counseling was provided before starting parisiticide treatment; all the children whose mothers were serologically positive were called to be tested; Chagas patients were performed complete check-ups and medical records were drawn; parasiticide treatment was prescribed for those patients that needed it, and informative meetings on Chagas Disease were held at the CAPS.
Both children older than 10 months of age and women of childbearing age were diagnosed by means of two serological determinations in venous blood: ELISA and HAI (indirect hemagglutination) and assessed prior to the beginning of treatment with hemogram, transaminase and urea.
All patients were administered 5 mg/kg/day of benznidazole, 400 mg maximum a day. At the beginning of the treatment, a pillbox with the two doses needed to complete the weekly treatment, a follow-up sheet to tick the dose taken, instructions with sanitary and diet tips to follow during treatment, details of the likely presence of adverse effects and what to do when said events take place were provided to the patients. In the case of those patients whose domicile was located far from the health care center and/or those who presented with previous allergy backgrounds were prescribed anthihistaminics, with the indication of the doses to be taken if necessary before attending the health care center for evaluation and control.
All patients were called for weekly appointments during the first month for supervision of the drug taken and to have it replaced, and to detect likely adverse effects as well. During the second month the interviews were made fortnightly. The design contemplated active domicile search on the part of the health agents and/or social workers of the patients that did not attend the appointment with the physician. Patients older than 21 years of age with no demonstrated pathology or incipient cardiopathy were given information on the levels of evidence and recommendation on how to receive treatment, as well as the results of preliminary studies regarding the benefits of the parasitic drug on reduction of the progression of the disease, so that they could opt whether to be treated or not. 20-21
In the case of people older than 50 years of age, such as the national standards in force state, it was decided not to start any treatment as a result of the lack of evidence of therapeutical effects and a greater likelihood to present complications with the specific medication.
Results
During the development of the pilot trial, until late 2013, a populational group considered at potential high risk of infection was assessed. Said group was constituted by 456 people: 129 children -between 2 and 15 years of age- and 329 adults. Of them, 181 presented positive serology for CD and received treatment.
The number of people diagnosed and treated increased at an annual rate as the working methodology was consolidated. This evolution can be observed in the graph, where, between the years 2011 and 2012 it rose by 25%, and for the year 2013 the annual rise regarding the previous year was 82%.
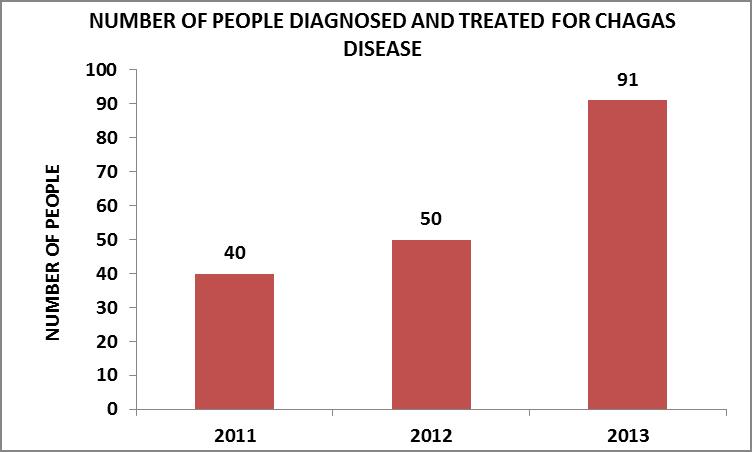
Source: Own elaboration
Most of the people diagnosed and treated were adult women who accounted for 78% of the total figure (144 women), 10% corresponded to adult men (18 men), and the children younger than 15 years of age were 12% with a similar distribution by sex.
The preponderance of females over males can be accounted for by the working methodology used, where focus was made on the survey on pregnant women, with gynecologists and obstetricians being in charge of diagnosis and therapeutic treatment.
Regarding treatment, all the patients opted to receive the parasiticide medication and none of them failed to have the controls made. Only three people older than 40 years of age (1.5%) had to interrupt the treatment since on two occasions they were undergoing a pruritic maculopapular rash that was quickly reversed with antihistaminics. They accomplished less than 30 days of medication treatment. These episodes took place at the beginning of the program of activities. In later years, only 35 patients (19.33%) presented with minor or moderate skin reactions that quickly reversed and did not represent a cause for interruption of the medication treatment.
No person under treatment did present severe skin reactions that demanded referral to higher complexity health centers and/or admission to hospital, neither did they present any of the other likely complications described.
All the people older than 15 years of age (160) were requested to have an ECG and cardiology control made. A total figure of forty-two (26.26%) people did not have these tests performed since they said they could not attend due to work commitments and since they lived in remote areas.
Limitations and Lessons Learned
The pilot trial became highly relevant in order to establish adjustments to the initial project. Both the risk survey and the informed consent revealed the need to incorporate to the final design complementary actions to be implemented due to the finding of a high number of parents with low levels of education.
Schools became the proper place to capture a high number of school-children in the short term and to guide parents; however, access to these facilities demanded strong articulation efforts beforehand.
Given the reach that prevention can have in this environment, it is important to re-think and strengthen the necessary bond between health and education areas to make prevention smoother.
Design in the sanitary environment contemplated a working modality that would prevent patients from being unnecessarily referred to the greater urban centers, given the distance to and from there and the difficulty in having regular public transport available. In order to do this, it was necessary to join the efforts of an important number of professionals (nurses, biochemists, obstetricians, pediatricians, health agents, social workers, midwives, social communicators, drivers and staff), whose commitment and dedication grew as time went by, driven by the results obtained.
When the CAPS’s resolution capacity was exceeded, patients were referred to levels of greater complexity, though attendance there was lower than that to the CAPS. One example of this was a cardiology consultation made, which had to be referred to greater complexity levels since there was no electrocardiograph and/or cardiologist available at the first level of care. The following are some of the reasons that account for a smaller attendance to other centers: distance, lack of public transport, and the type of daily job that leave workers little margin of free time to make consultations. It is also worth mentioning that in many health centers that are not integrated into this project, patients were given contradictory messages on the need to carry out parasitic idée treatment, or the practices they had been requested at the CAPS were rejected. It is for those reasons that, in many cases, patients opted not to continue demanding for treatment.
The therapeutically strategy established -similar to DOT, directly observed therapy- enabled health teams and patients to have an important tool available when they needed to make early supervision, detection and treatment of outcomes of adverse reactions. When these appeared, they were minor or moderate and ceased with oral antihistaminic medication.
While this project was being implemented, benznidazole production and supply were interrupted, which obviously delayed both the development of the project and the speed to capture and treat patients, facts that were reversed when supply was re-started.
Initially, and resulting from the methodology for patient selection, the adult population was exclusively female. During the last year, some men started to make spontaneous consultations to be diagnosed and treated, although the figure is low in relation to women’s.
While training the health teams, it was observed that even though Chagas disease is well known, the therapeutic experience is null and the initial fears of the apparent adverse effects caused by the drugs are relatively high. The strategies adopted (directly observed treatment, the possibility of permanent telephone communication of the physician in charge of the treatment with national specialist on the issue, and the availability of the drugs to control likely complications) were important at the beginning until the team achieved therapeutic experience. It is highly probable that allocation of more teaching hours devoted to diagnosis and treatment of Chagas disease within physiciansâ training curricula will help generate greater commitment on the part of the health teams to these issues.
Discussion
Being able to diagnose and treat newborns and children, as well as women at childbearing age when they have not shown any manifestations of the disease yet should be a priority within the health environments, especially for those in charge of health policy making and management, considering the results of said actions and their cost-benefit.
This work makes it possible to observe how active policies and an adequate epidemiological assessment can generate important findings in a relatively small and unthought-of area, and deploy prevention and cure actions.
It also expresses clearly the need to strengthen the first level of care to face this issue and the need to generate patient-centered models of management of chronic pathologies that can help overcome the barriers to access to a health system, usually suffered by the people affected who live in remote areas.
Acknowledgements
The authors would like to thank Dr. Héctor Freilij for the training offered to our professionals, his valuable contributions, and the enthusiasm he transmitted carrying out his work; and Dr. Gustavo Marin for having driven us to start the activities in the La Plata district
References
1– Corallini, JC; Fernandez, O; Della Vedova, A; Dicroce, MH; Bianconi, M; Gonzalez, MR; Puyou, NE; Gallinger, CG; Salvo, SA; Martin, MS; Tedeschi, MM; Gargiulo, ML; Zoraida Correa, S; Abadie, MS y Arguiano, SE (2011): Enfermedad de Chagas-Mazza: seroprevalencia, caracteristicas epidemiologicas y sociales. Acta Bioquimica Clinica Latinoamericana, Federacion Bioquimica de la Provincia de Buenos Aires, vol. 45, num. 3, pp. 431-439. Argentina. http://www.redalyc.org/pdf/535/53521520004.pdf
2-Toso, Alberto; Vial, Felipe; Galanti, Norbel (2011): “Transmision de la enfermedad de Chagas por via oral”. Rev Med Chile 2011; 139: 258-266. Santiago de Chile.
3– Conrado M. Cusnaider y col. (2004): “Chagas congenito es posible en Espana?” Ginecologia y Obstetricia Clinica 2004; 5(4):198-203.
4– Basile L, et al. (2011): “Working Group on Chagas Disease. Chagas disease in European countries: the challenge of a surveillance system” . Euro Surveill. , 2011; 16(37):pii=19968.
5– Blood Donor Screening for Chagas Disease — United States, 2006â2007 CDC February 23, 2007 / 56(07);141-143.
6– Kirchhoff, Louis V. ; Pearson, Richard D (2007): “The emergence of Chagas disease in the United States and Canada”, Current Infectious Disease Reports, Vol. 9,Nº5, Sep 2007.
7– Altcheh J (2013): Enfermedad de Chagas en la Infancia. Presented at the Discussion Workshop “Chagas: enfermedad atendida” (Chagas: a disease cared for) Fundacion Mundo Sano, Buenos Aires, 3 June 2013.
8– Zaidenberg, M (2009) “Chagas en ninos. El desafio de los ultimos tiempos” (Chagas in children. The Challenger of recent times) presented at the 12th International Symposium of Epidemiological Control of vector-borne diseases. Fundacion Mundo Sano, Buenos Aires, 4 September, 2009.
9– PAHO Webpage http://www.paho.org/hq/index.php?option=com_topics&view=article&id=10&Itemid=40743&lang=en
10-PAHO (2006): “Estimacion cuantitativa de la enfermedad de Chagas en las Americas”. Montevideo, Uruguay. OPS/HDM/CD/425-06; 2006. p. 1-28.
11-PAHO- Mundo Sano (2007): La enfermedad de Chagas a la puerta de los 100 anos del conocimiento de una endemia americana ancestral. OPS/CD/426-06 Mundo Sano. July 2007. Argentina
12-2011 Vital Statistics. Directorate of Health Statistics and Information. Ministerio de Salud de la Nacion. Available at http://www.deis.gov.ar/Publicaciones/Archivos/Serie5Nro55.pdf
13-Chuit, R, Segura, EL (2012). “El control de la enfermedad de Chagas en Argentina. Sus resultados”. Rev. Fed. Arg. Cardiol 2012;41(3):151-155
14-Ministerio de Salud de la Nacion (2010). Guia de diagnostico y tratamiento. Informacion para los equipos de salud. Direccion de Epidemiologia. Programa Nacional de Chagas. Available at http://www.msal.gov.ar/chagas/index.php/informacion-para-equipos-de-salud/guia-de-diagnostico-y-tratamiento
15-OPS (2012) II Reunion Sudamericana de Iniciativas Subregionales de Prevencion, Control y Atencion de la Enfermedad de Chagas. OPS, August 2012.
16-Ministerio de Salud de la Nacion (2010). Guia de diagnostico y tratamiento. Informacion para los equipos de salud. Direccion de Epidemiologia. Programa Nacional de Chagas. Available at http://www.msal.gov.ar/chagas/index.php/informacion-para-equipos-de-salud/guia-de-diagnostico-y-tratamiento
17-Estadisticas. Direccion Nacional de Migraciones Ministerio del Interior y Transporte http://www.migraciones.gov.ar/accesible/?estadisticas
18-Pizzolitto, G; Porto, A; (2006): “Distribucion de la poblacion y migraciones internas en Argentina: sus determinantes individuales y regionales”. Universidad Nacional de La Plata; Facultad de Ciencia Economicas. December 2006
19-“Encuesta Perinatal 2008: Resultados en Hospitales Publicos de la Provincia de Buenos Aires y Ciudad Autonoma de Buenos Aires.” Page 66. Available at http://salud.ciee.flacso.org.ar/files/flacso/AMBA/pdf/OBLIGATORIA/1semEncuestaPerinatal2008.pdf
20-Sosa Estani, S; Segura, E; Colantonio, L (2012): “Therapy of Chagas Disease: Implications for Levels of Prevention”. J Trop Med. 2012; 2012: 292138
21-Viotti, R; Vigliano, C; Lococo, B; Bertocchi, G; Petti, M; Alvarez, MG; Postan, M; and Armenti, A (2006): “Long-Term Cardiac Outcomes of Treating Chronic Chagas Disease with Benznidazole versus No Treatment A Nonrandomized Trial” ;Ann Intern Med. 2006;144:724-734.