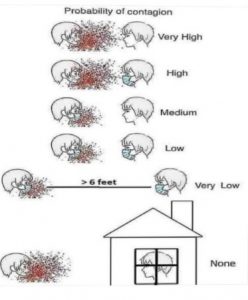
In this era of the COVID-19 pandemic, norms have been upended in every aspect of society. A lesser known complication in the healthcare environment is the impact on clinical trials. There is an opportunity to rethink how trials are conducted and also implement much-needed changes for greater inclusion of diverse trial participants by building on the momentum of developments started prior to the Coronavirus pandemic. The scope of this review will focus on rural study subjects and their barriers to participation
By Nicole Jarosinski, MA
AllBe Health, Amsterdam, The Netherlands
The Future Path of U.S. Clinical Research: Bridging Geographic Diversity
In this era of the COVID-19 pandemic, norms have been upended in every aspect of society. The media has paid particular attention to the challenges and demands on healthcare around the world as hospitals navigate a surge in patient census, pause elective procedures, and otherwise cope with new realities. A lesser known complication in the healthcare environment is the impact on clinical trials. Trials determine the treatments and best practices of the future. With the ongoing changes in healthcare and society at large, there is an opportunity to rethink how trials are conducted and also implement much-needed improvements for greater inclusion of diverse trial participants by building on the momentum of developments started prior to the Coronavirus pandemic.
In 2019, the US Food and Drug Administration (FDA) issued guidance[1] calling for greater diversity of clinical trial populations with the purpose of developing a full picture of the risk or benefit of an investigational medical product. Further, “experience has shown that there can be important differences in how people of diverse groups respond to medical products. Information on those differences can then be included in the product labeling to help doctors and patients make treatment decisions.”[2] Given that the majority of clinical trial participants in the United States tend to be affluent white males in urban and suburban locations, diversity in this context means more than racial factors. A diverse clinical trial would also include different ages, ethnic groups, genders, and geographic settings. Participants with diverse characteristics have vital information to contribute to a more complete scientific understanding of medicine. The scope of this review will focus on rural study subjects and their barriers to clinical trial participation. Rural populations are historically underrepresented in United States trials and face the possibility of being even less represented in this time of COVID.
Challenges of rural populations
Rural populations in the United States share a number of common challenges. According to the Pew Research Center, residents of rural areas have lower income and rates of employment.[3] They have problems accessing fast, reliable internet service, with 22% saying they never go online.[4] Rural Americans are also older, sicker, and less likely to be insured than their suburban and urban counterparts.[5] Twenty percent of Americans live in rural areas, but the proportion participating in clinical trials is far less. Fewer than five percent of studies funded through the National Institutes of Health National Cancer Institute (NIH NCI) demonstrate recruitment in rural populations.[6] Given these challenges, there are a number of reasons that rural populations are often overlooked as potential sources of research participants.
The Devil You Know
Sponsors tend to choose academic medical centers located in major metropolitan areas as study sites due to their dedicated investigative resources and research-based mission. Academic centers have reputations for cutting-edge medicine that also make them destination hospitals, drawing patients from across the country. It is expensive for a clinical trial sponsor to prepare sites to conduct clinical trials due to multiple on-site visits, inspections, and training. It’s not uncommon for a sponsor to invest $30,000 in each site at startup, but this investment does not guarantee that a site will ever enroll any subjects. Therefore, sponsors often return to sites that have delivered high enrollment and complete data in past studies. Sponsors are also reluctant to contract with new sites that may not have as large of a patient pool.
Transportation Roadblocks
Reliable transportation is a barrier for rural populations to participate in trials that have a heavy number of in-person follow-up visits. These types of visits have their advantages, including more complete data capture. In-person visits also allow an unbroken chain of custody for investigational drugs that are literally handed from the research team to the patient, thus eliminating the possibility of restricted medications being lost in the mail. Despite these advantages, on-site visits can place a heavy travel demand on study subjects. Demands are exacerbated for those who must traverse long distances, those who need assistance, such as the elderly or children, and those who have to miss work or other obligations in order to participate.
Technical Difficulties
Telehealth technologies that could potentially reach rural populations have been slow to develop in the United States. One barrier to telehealth is the lack of highspeed internet in rural communities, but arguably the biggest factor for slow telehealth development has been non-involvement from the Centers for Medicare & Medicaid Services (CMS). CMS is the largest payor of medical claims in the US and greatly influences the direction of healthcare. Even though telehealth technology was developed in the early 2000s, CMS strictly limited telehealth reimbursement until 2018. Without financial support from CMS, healthcare providers had little incentive to make investments in telehealth.
Overcoming the Challenges
Increasing participation of rural populations in clinical trials requires a three-pronged approach: 1) expanding the network of potential trial sites; 2) improving participants’ access to healthcare; and 3) providing flexibility for the method and frequency of trial follow-up visits.
Broader Site Networks
Reputation is important, so it is understandable that sponsors would return to high-enrollment research sites that they contracted with in the past. However, to meet FDA expectations of diverse enrollments, it is imperative for sponsors to extend their reach into new territories. To facilitate expanded connections in rural areas, sponsors can partner with organizations who have existing networks, such as the NCI Community Oncology Research Program (NCORP). With a mission to bring “cancer clinical trials and care delivery studies to people in their own communities,” NCORP is already conducting trials in rural and underserved areas.[7] By collaborating with NCORP or similar entities, sponsors may find new long-lasting partners while also bringing cutting edge treatments to areas of the country that otherwise wouldn’t have access.
Clearing the Road
Social infrastructure improvements in recent years have increased the mobility of rural populations. Clinical trial sponsors and sites can take advantage of these enhancements for patients that struggle with travel. For short distances, ridesharing services such as Uber and Lyft can bring patients to research sites. Lesser known services that specifically serve rural populations are shuttles offered by local counties. Community-based councils on aging or volunteers from elder care and/or disabled advocacy groups also offer rides to medical appointments for study participants who would otherwise be homebound. These agencies may offer multi-county transport options at low to no cost.
Travel reimbursement can ease the burden of trial participation for all subjects regardless of distance from the study site, and it is a small cost to promote study enrollment. The FDA has clarified that it “does not consider reimbursement for travel expenses to and from the clinical trial site and associated costs such as airfare, parking, and lodging to raise issues of undue influence.”[8] Such reimbursements are generally considered acceptable practice.
Another unique solution is to bring studies to rural subjects through collaboration with healthcare services that already interact with patients in remote settings. As an example, university researchers in Arkansas found great success in recruiting rural and minority participants by joining forces with traveling care teams, such as mobile mammography units. They also recruited by participating in community events, such as the Susan G. Komen Race for the Cure, church functions, and health fairs.[9]
Streamlined Follow-ups
There are many common sense and innovative ways sponsor can rethink the strategy for a follow-up visit schedule. First, reduce the frequency of visits to only those necessary for appropriate safety and efficacy monitoring. Not only would this reduce the burden of study participation for research subjects, it would also decrease costs for sponsors.
Another factor is to build flexibility for the method of follow-up visits so that they could be conducted at the research site, the patient’s home, or virtually. There are an increasing number of remote and mobile communication platforms that can accommodate clinical trials. Providing multiple options makes study participation more convenient to patients.
Finally, research sites can take advantage of incentive programs for the expansion of telehealth. The Federal Communications Commission (FCC) recently announced that it is offering telehealth startup funding through the COVID-19 Telehealth Program and Connected Care Pilot Program.[10] These programs will lower the initial investment needed from study sites to build telehealth infrastructure.
The Path Forward
Historically, the pharmaceutical industry has been extremely conservative, changing very slowly due to its risk-averse nature. However, clinical research is in tumultuous times as sponsors and sites hurry to figure out the path forward through complications brought on by COVID-19. It has been said that necessity is the mother of invention. In the midst of such great change there is also vast opportunity.
Sponsors can easily build on the momentum of recent innovations to both adapt ongoing clinical trials to the new 6-foot (1.5 meter) society and be more inclusive of rural participants. In fact, the above recommendations could be easily translated to increase diversity of all kinds, since concerns about transportation and low income span across all races, genders, ages, and geographic locations.
There will inevitably be speed bumps for a while as new best practices are established, technology is refined, and new bridges are crossed. But for the clinical trial industry, the COVID-19 pandemic is an inflection point. There is no going back to the way it has always been done. Patience, ingenuity, and a willingness to embrace change are what is needed in this time.
References
[1] U.S. Department of Health and Human Services, Food and Drug Administration. (2019). Enhancing the diversity of clinical trial populations: Eligibility criteria, enrollment practices, and trial designs. Draft Guidance. Retrieved from https://www.fda.gov/media/127712/download.
[2] U.S. Department of Health and Human Services, Food and Drug Administration. (2018). FDA encourages more participation, diversity in clinical trials. Retrieved from https://www.fda.gov/consumers/consumer-updates/fda-encourages-more-participation-diversity-clinical-trials.
[3] Parker, K., Horowitz, J.M., Brown, A., Fry, R., Cohn, D., & Igielnik, R. (2018). What unites and divides urban, suburban and rural communities: Amid widening gaps in politics and demographics, Americans in urban, suburban and rural areas share many aspects of community life. Pew Research Center. Retrieved from https://www.pewsocialtrends.org/2018/05/22/what-unites-and-divides-urban-suburban-and-rural-communities/.
[4] Anderson, M. (2018). About a quarter of rural Americans say access to high-speed internet is a major problem. Pew Research Center. Retrieved from https://www.pewresearch.org/fact-tank/2018/09/10/about-a-quarter-of-rural-americans-say-access-to-high-speed-internet-is-a-major-problem/.
[5] U.S. Centers for Disease Control and Prevention (2017). About Rural Health. Retrieved from https://www.cdc.gov/ruralhealth/about.html#:~:text=A%20series%20of%20studies%20from,stroke%20than%20their%20urban%20counterparts.
[6] Blake, K.D., Moss, J.L., Gaysynsky, A., Srinivasan, S., & Croyle, R.T. (2017). Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 26, 992-997.
[7] National Cancer Institute Community Oncology Research Program. (n.d.). About NCORP. Retrieved from https://ncorp.cancer.gov/about/.
[8] U.S. Department of Health and Human Services, Food and Drug Administration. (2018). Payment and reimbursement to research subjects: Guidance for institutional review boards and clinical investigators. Final Information Sheet. Retrieved from https://www.fda.gov/RegulatoryInformation/Guidances/ucm126429.htm.
[9] McElfish, P.A, Su, L.J., Lee, J.Y., Runnells, G., Henry-Tillman, R., & Kadlubar, S.A. (2019). Mobile Mammography Screening as an Opportunity to Increase Access of Rural Women to Breast Cancer Research Studies. Breast Cancer: Basic and Clinical Research, 13, 1-6. DOI: 10.1177/1178223419876296.
[10] U.S. Federal Communications Commission. (2020). FCC Approves Emergency COVID-19 Telehealth and Connected Care Pilot Programs. Retrieved from https://www.fcc.gov/fcc-approves-emergency-covid-19-telehealth-and-connected-care-pilot-programs.